

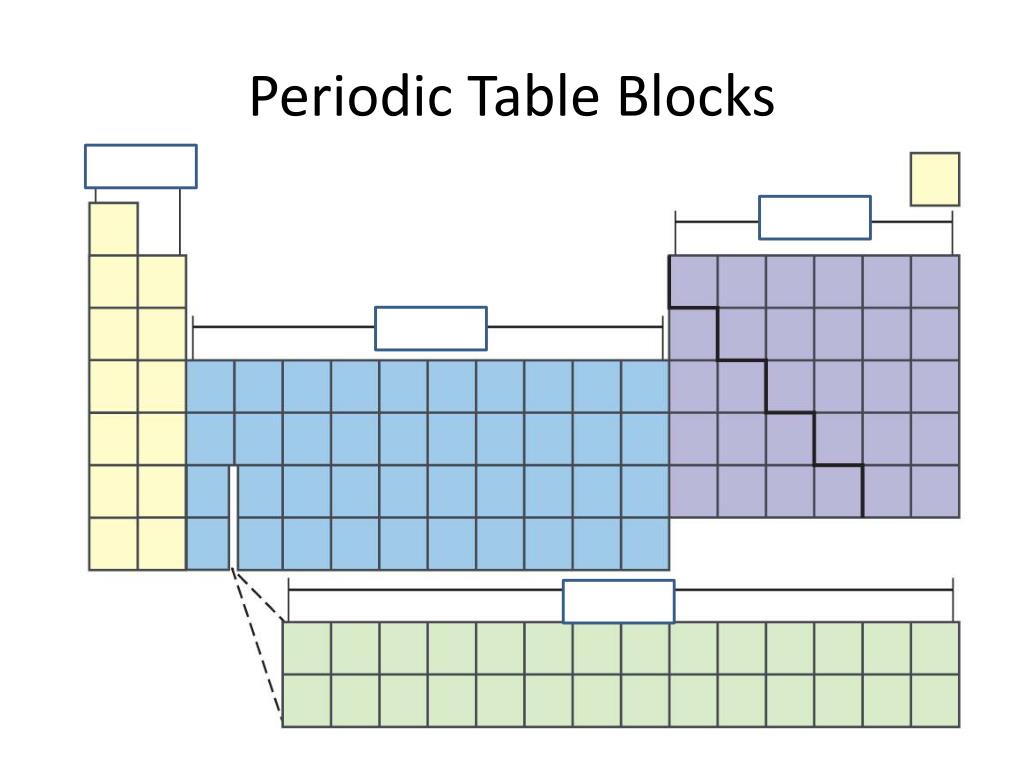
However, they remain d-block elements even when considered to be main group. The group 3 elements are occasionally considered main group elements due to their similarities to the s-block elements. The group 12 elements zinc, cadmium, and mercury are sometimes regarded as main group, rather than transition group, because they are chemically and physically more similar to the p-block elements than the other d-block elements. The s-block and p-block together are usually considered main-group elements, the d-block corresponds to the transition metals, and the f-block corresponds to the inner transition metals and encompasses nearly all of the lanthanides (like lanthanum) and the actinides (like actinium). There is an approximate correspondence between this nomenclature of blocks, based on electronic configuration, and sets of elements based on chemical properties. Useful statements about the elements can be made on the basis of the block they belong to and their position in it, for example highest oxidation state, density, melting point… Electronegativity is rather systematically distributed across and between blocks. The division into blocks is justified by their distinctive nature: s is characterized, except in H and He, by highly electropositive metals p by a range of very distinctive metals and non-metals, many of them essential to life d by metals with multiple oxidation states f by metals so similar that their separation is problematic.


 0 kommentar(er)
0 kommentar(er)
